CDC, Vaccines from 6 months
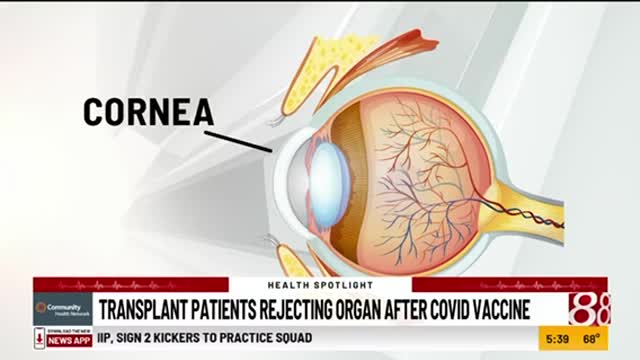
C.D.C. Recommends 2 Covid Vaccines for under 5s ONS, https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/antibodies 95.9% of children aged 8 to 11 years had antibodies against SARS-CoV-2 Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children Risks of Myocarditis and Pericarditis The FDA and CDC safety surveillance systems have previously identified increased risks of myocarditis and pericarditis following vaccination with the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, particularly following the second dose. The observed risk is highest in, males 18 through 24 years of age for the Moderna males 12 through 17 years of age for the Pfizer-BioNTech COVID-19 Vaccine. most cases of myocarditis associated with the Moderna and Pfizer-BioNTech COVID-19 vaccines are characterized by rapid resolution of symptoms with no impact on quality of life reported by most patients who were contacted for follow-up at 90 days or more after reporting myocarditis. https://www.nytimes.com/live/2022/06/18/world/covid-19-mandates-vaccine-cases 19 million Moderna for ages 6 months through 5 years Pfizer-BioNTech ages 6 months through 4 years Dr. Rochelle P. Walensky, the C.D.C.’s director All children 6 months and older, including those who have already been infected with the coronavirus, should get a Covid vaccine Together, with science leading the charge, we have taken another important step forward in our nation’s fight against Covid-19 We know millions of parents and caregivers are eager to get their young children vaccinated, and with today’s decision, they can Albert Bourla, Chairman and Chief Executive Officer, Pfizer We know many parents in the U.S. have been eagerly awaiting an authorized vaccine for their children under 5 and we are proud to now offer them a vaccine option with a favorable safety profile Pfizer-BioNTech COVID-19 Vaccine Demonstrates Strong Immune Response, High Efficacy and Favorable Safety in Children 6 Months to Under 5 Years of Age Following Third Dose https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-covid-19-vaccine-demonstrates-strong-immune Vaccine efficacy was 80.3% in children 6 months to under 5 years of age. N = 1,678 had 3 doses, (omicron times) This descriptive analysis was based on 10 symptomatic COVID-19 cases identified from seven days after the third dose and accrued as of April 29, 2022. (3 in the vaccine group, 7 in the placebo group) The trial protocol specifies a formal analysis will be performed when at least 21 cases have accrued from seven days after the third dose. Final vaccine efficacy data will be shared once available. Pfizer-BioNTech COVID-19 Vaccine Receives FDA Emergency Use Authorization for Children 6 Months through 4 Years of Age https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-covid-19-vaccine-receives-fda-emergency-use MODERNA ANNOUNCES ITS COVID-19 VACCINE PHASE 2/3 STUDY IN CHILDREN 6 MONTHS TO UNDER 6 YEARS HAS SUCCESSFULLY MET ITS PRIMARY ENDPOINT https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-its-COVID-19-Vaccine-Phase-23-Study-in-Children-6-Months-to-Under-6-Years-Has-Successfully-Met-Its-Primary-Endpoint/default.aspx 6 months to 2 years, n = 2,500 enrolled 2 to under 6, n = 4,200 enrolled Rates of fever post vaccine, greater than 38 6 months to 2 years, 17% 2 years to under 6, 14.6% 6 to 12 years, 23.9% Vaccine efficacy Similar antibody response to in 18- to 25-year-olds 6 months to 2 years, 43.7% 2 to under 6 years, 37.5% Kaiser Family Foundation survey https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-april-2022/ Plan to get under 5s vaccinated, 18% Definitely not, 27% Tom Inglesby, Johns Hopkins Center for Health Security, Bloomberg School of Public Health There’s a lot of information and trust building that needs to happen Hopefully, with time, people will have increasing confidence that it’s both effective and safe Neither vaccine was tested against BA.4 and BA.5 (exceptional immune escape) Peter Hotez, molecular virologist, National School of Tropical Medicine at Baylor College of Medicine, Houston The pediatric vaccines, won’t hold up that well in protecting against infection by the new subvariants But they will still be very effective in preventing children from going to the hospital or the intensive care unit ACIP Committee Members https://www.cdc.gov/vaccines/acip/members/index.html